Water Quality and Corrosion Study
Water quality and the protection of public health are our top priorities. In late 2018, we completed a two-year Water Quality and Corrosion Study to better understand options to further reduce the possibility of lead and copper leaching from materials in the water system and home plumbing. Our two water supplies – Big Butte Springs and the Rogue River – do not contain lead or copper.
Why did Medford Water study corrosion?
The ongoing lead issues for customers across the nation highlight the need for water systems to take the issue of reducing lead exposure seriously.
Medford Water conducted a two-year Water Quality and Corrosion Study to evaluate treatment options that can reduce the water’s tendency to release metals from service lines and household plumbing. Medford Water is in compliance with all Environmental Protection Agency (EPA) regulations.
However, there may be an opportunity to create a more stable water, allowing us to deliver the highest quality water to customers’ taps.
The study started in May 2017 and was completed an independent expert team to identify a scientifically rigorous and credible solution. All work is based on the latest guidance from the EPA.
The study is part of a multi-pronged approach to lead and copper in the water system. Several years ago, Medford Water worked to proactively find and replace lead pipe “pigtails” installed in the early 1900s. For more information on those efforts and information you can take to reduce your exposure to lead and copper in drinking water, click here.
What is the best way to preserve Medford Water’s water quality?
The Water Quality and Corrosion Study evaluated the best path forward to preserve Medford Water’s high quality water all the way to the tap. The thorough four-phase approach followed EPA best practices and assures that any treatment chosen will work with our two water sources and piping system. It has illustrated how much the water quality will improve and estimate how much corrosion treatment will cost. The study included four phases, described in the below graphic.
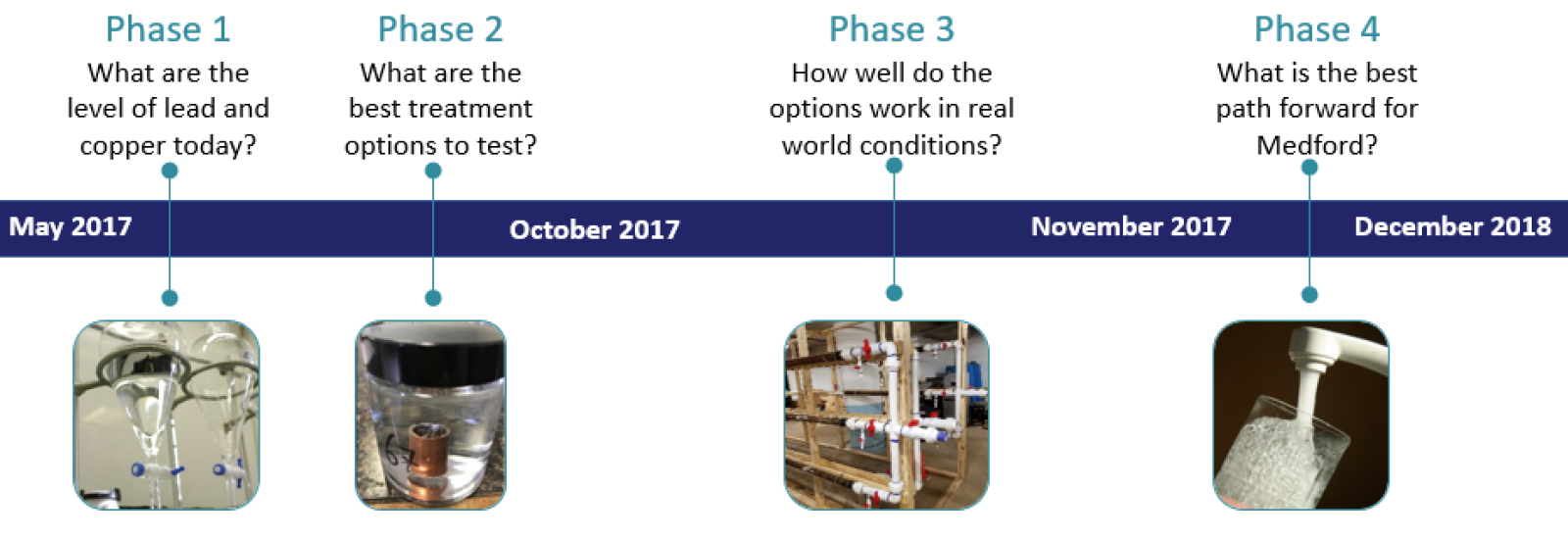
What have we learned so far?
|
What is pH?
· pH is a scale of acidity from 0-14:
acidic (low pH) versus alkaline
(high pH)
· Mid-point on the scale is 7 (neutral)
· Higher on pH scale: baking soda, toothpaste, hand soap
· Lower on pH scale: lemon juice,
vinegar, black coffee
· Drinking water with lower pH has a
greater tendency to release lead and
copper from piping and household
plumbing. Water with a higher pH
is less corrosive.
|
The results show that increasing the pH (making water less corrosive) produces a more stable water that reduce the tendency of metals to leach from pipes. That means that regardless of the type of pipes people have in their homes, with pH treatment, metals stay in the pipe instead of leaching into the water. The pH adjustment methods add naturally occurring substances that have been safely used for decades in communities across Oregon and the U.S.
These treatment methods were then tested in “pipe loops”, using real pipe from our system to assure that any treatment chosen will work with our two water sources and piping system.
This process identified that adjusting the water chemistry with sodium hydroxide is the best option to reduce the release of lead and copper. Sodium hydroxide is used at thousands of drinking water plants across the nation; when dissolved in water, it breaks down into sodium (found in table salt) and hydroxide ions (found in all water). The sodium concentration in the water will remain at what is considered a very low level by drinking water guidelines.
What are the next steps of the study?
Now that we have identified the best option to preserve our community’s high-quality water right to the tap, we are working to design and build the facilities needed to implement this treatment. The pH change is anticipated to take place in early 2024.
How can I learn more?
For questions, contact Water Quality at 541-774-2430.
|